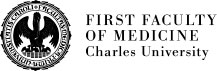Regeneration of hematopoietic tissue
Hematopoietic tissue provides a unique opportunity to study tissue regeneration due to its well established steady-state structure and function, easy accessibility, well established research methods, and the well-defined embryonic, fetal, and adult stages of development. Hematopoietic tissue regeneration is believed to start by activation of dormant stem cells. However, this process is relatively poorly understood, and the question of how the very rare stem cells can rapidly increase their own pool concomitantly with the production of life-saving blood cells still remains unanswered. Clearly the processes like proliferation/quiescence and self-renewal/differentiation must be tightly controlled.
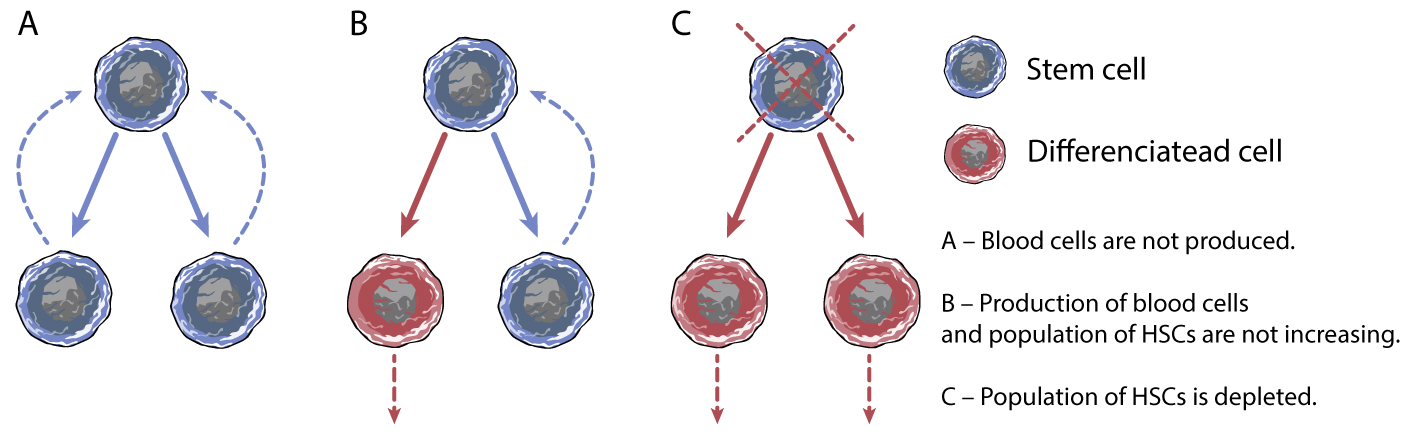
Embryonic/fetal liver hematopoiesis and adult hematopoiesis when recovering from damage have in common the necessity to expand populations of progenitors and stem cells in parallel with immense production of mature blood cells. We analyze adult hematopoiesis in mice subjected to a submyeloablative doses (3 or 6 Gy) of gamma radiation and analyze subsequent periods of regeneration. In the period of regeneration characterized by massive production of mature blood cells along with ongoing expansion of immature hematopoietic cells, we uncovered significantly expanded populations of altered erythro-myeloid progenitor cells which are c-Kit low , partly express Sca-1 and the majority of them express CD16/32. This cell phenotype is in marked contrast to the steady-state adult hematopoiesis and to the expanding hematopoiesis in the fetal liver and postnatal bone marrow.
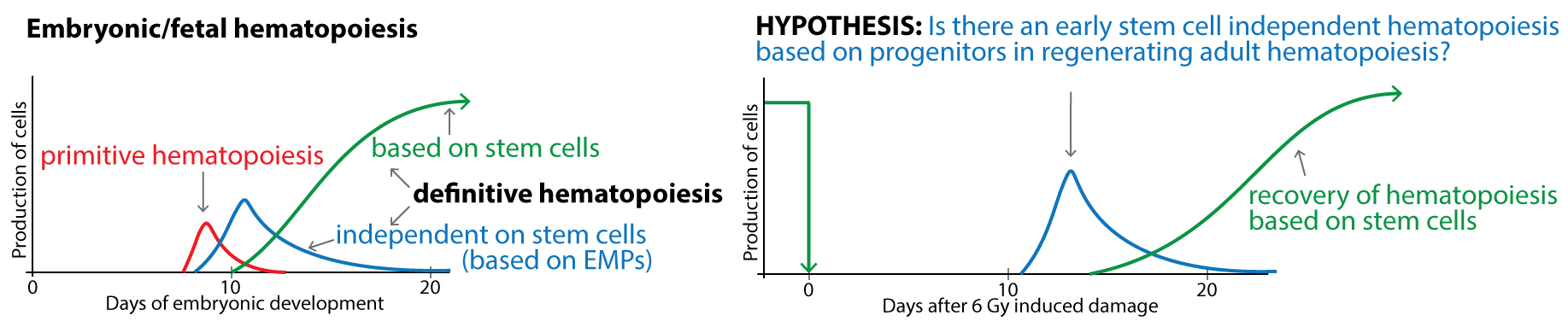
However, regenerating hematopoiesis shares some similarities with the second wave of embryonic hematopoiesis driven by specific erythro-myeloid progenitor cells (EMP; McGrathet al., 2015) and lacking hematopoietic stem cells. Both, the regenerating hematopoiesis and the second wave of embryonic hematopoiesis are characterized by 1) concomitant population expansion of myeloid progenitors and increasing production of myeloid blood cells 2) performing these tasks despite the severely reduced transplantation capacity 3) CD16/32 expression on progenitor cells.
We suggest that cells other than stem cells and multipotent progenitors can be of fundamental importance for the rapid recovery of function in damaged tissue prior to the reestablishment of the normal structure of hematopoietic tissue with stem cells at the apex of thehematopoietic hierarchy.
One of the current focuses of our lab is to uncover the molecular and biological mechanisms involved in the spontaneous regeneration of hematopoiesis after its damage by submyeloablative doses of ionizing radiation.