Petr Páral, MSc., PhD
Topic:
Developmental Hierarchy of Hematopoietic Stem and Progenitor Cells and Their Regulation in Bone Marrow.

 www.researchgate.net/profile/Petr_Paral
www.researchgate.net/profile/Petr_Paral
Petr Páral Publications
Cell Cycle Analysis Using In Vivo Staining of DNA-Synthesizing Cells.
Páral P., Báječný M., Savvulidi F., Nečas E. (2019), Methods Mol Biol. doi: 10.1007/7651_2019_228.
Abstract:
The thymidine analogues BrdU (5-bromo-2´-deoxyuridine) and EdU (5-ethynyl-2´-deoxyuridine) are routinely used for determination of the cells synthesizing DNA in the S-phase of the cell cycle. Availability of the anti-BrdU antibody clone MoBu-1 detecting only BrdU allowed to develop a method for the sequential DNA labelling by these two thymidine analogues for determining the cell cycle kinetic parameters.In the current step-by-step protocol, we present` two approaches optimized for in vivo study of the cell cycle and the limitations that such approaches imply: (1) determination of the cell flow rate into the G2-phase by dual EdU/BrdU DNA-labelling method and (2) determination of the outflow of DNA-labelled cells arising from the mitosis.
Cell cycle and differentiation of Sca-1+ and Sca-1- hematopoietic stem and progenitor cells.
Páral P., Faltusová K., Molík M., Renešová N., Šefc L., Nečas E. (2018), Cell Cycle. doi: 10.1080/15384101.2018.1502573.
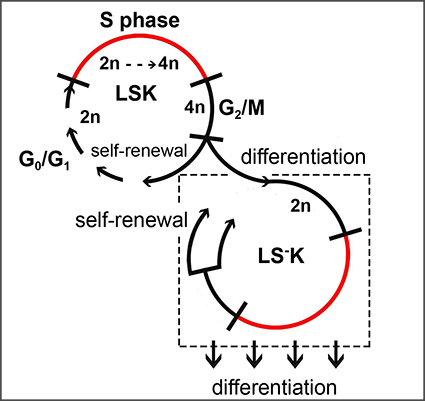 Abstract: Abstract:Hematopoietic stem and progenitor cells (HSPCs) are crucial for lifelong blood cell production. We analyzed the cell cycle and cell production rate in HSPCs in murine hematopoiesis. The labeling of DNA-synthesizing cells by two thymidine analogues, optimized for in-vivo use, enabled determination of the cell cycle flow rate into G2-phase, the duration of S-phase and the average cell cycle time in Sca-1+ and Sca-1- HSPCs. Determination of cells with 2n DNA content labeled in preceding S-phase was then used to establish the cell flow rates in G1-phase. Our measurements revealed a significant difference in how Sca-1+ and Sca-1- myeloid progenitors self-renew and differentiate. Division of the Sca-1+ progenitors led to loss of the Sca-1 marker in about half of newly produced cells, corresponding to asymmetric cell division. Sca-1- cells arising from cell division entered a new round of the cell cycle, corresponding to symmetric self-renewing cell division. The novel data also enabled the estimation of the cell production rates in Sca-1+ and in three subtypes of Sca-1- HSPCs and revealed Sca-1 negative cells as the major amplification stage in the blood cell development. |
Hematopoiesis Remains Permissive to Bone Marrow Transplantation After Expansion of Progenitors and Resumption of Blood Cell Production.
Báječný, M., Chen C.-L.,Faltusová, K., Heizer T., Sziskszai K., Páral P., Šefc, L., and Nečas, E. (2021)., Front. Cell Dev. Biol., https://doi.org/10.3389/fcell.2021.660617
Altered erythro-myeloid progenitor cells are highly expanded in intensively regenerating hematopoiesis.
K. Faltusová, C-L. Chen , T. Heizer, M. Báječný, K. Szikszai, P. Páral, F. Savvulidi, N. Renešová and E.Necas (2020), Front. Cell Dev. Biol. doi: 10.3389/fcell.2020.00098
T-lymphopoiesis is Severely Compromised in Ubiquitin-Green Fluorescent Protein Transgenic Mice
K. Faltusová, M. Báječný, T. Heizer, P. Páral, E. Nečas (2020), FOLIA BIOL (PRAHA). Accepted.
Synthesis and modification of uniform PEG-neridronate-modified magnetic nanoparticles determines prolonged blood circulation and biodistribution in a mouse preclinical model.
V. Patsula, D. Horák, J. Kučka, H. Macková, V. Lobaz, P. Francová, V. Herynek, T. Heizer, P. Páral & L. Šefc, Sci Rep, 2019, doi: 10.1038/s41598-019-47262-w
Stem Cell Defect in Ubiquitin-Green Fluorescent Protein Mice Facilitates Engraftment of Lymphoid-Primed Hematopoietic Stem Cells.
Faltusová, K., Szikszai, K., Molík, M., Linhartová, J., Páral, P., Šefc, L., Savvulidi, F. and Nečas, E. (2018), STEM CELLS. doi: 0.1002/stem.2828.
Thermoresponsive β-glucan-based polymers for bimodal immunoradiotherapy – Are they able to promote the immune system?
L. Loukotová, J. Kučka, M. Rabyk, A. Höcherl, K. Venclíková, O. Janoušková, P. Páral, V. Kolářová, T. Heizer, L. Šefc, P. Štěpánek, M. Hrubý, j cont rel, 2017, doi: 10.1016/j.jconrel.2017.10.010
The pharmacological activation of adenosine A1 and A3 receptors does not modulate the long- or short-term repopulating ability of hematopoietic stem and multipotent progenitor cells in mice.
M. Hofer, M. Pospíšil, Z. Hoferová, D. Komůrková, P. Páral, F. Savvulidi, and L. Šefc, Purinergic Signal. 2013, doi: 10.1007/s11302-012-9340-5
Effects of endostatin production on oncogenicity and metastatic activity of HPV16-transformed mouse cells: role of interleukin 1alpha.
Lakatosova-Andelova M1, Duskova M, Lucansky V, Paral P, Vonka V., Int J Oncol. 2009, doi: 10.3892/ijo_00000331
Petr Páral Posters
Early erythroid differentiation is closely associated with the progression trough S-phase.
P. Páral, M. Báječný, K. Faltusova, T. Heizer, L. Sefc, E. Nečas. International Conference on Stem Cells, Crete 2019
Cell cycle dynamics and functional organization of immature hematopoietic cells.
P. Paral K. Faltusova, M.Molik, N. Renesova, L. Sefc, E. Necas. CYTO, Prague, 2018
Hierarchy of Sca-1positive and Sca-1negative hematopoietic stem and progenitor cells and their contribution to blood cell production.
P. Paral K. Faltusova, M.Molik, N. Renesova, L. Sefc, E. Necas. ISEH, Frankfurt, 2017
Sca-1negative haematopoietic progenitor cells markedly differ in proliferation rate and are vital for blood cell production.
P. Paral K. Faltusova, M.Molik, N. Renesova, L. Sefc, E. Necas. Lyon, 2016.
Oscillations in hematopoietic stem cell numbers and proliferation during recovery from cyclophosphamide treatment on murine model.
P. Paral, F. Savvulidi, E. Necas, L. Sefc. ISSCR, Stockholm, 2015.
Petr Páral PhD thesis
The cell cycle and differentiation of haematopoietic stem and progenitor cells.
Prague, 2019
Abstract:
Haematopoietic stem and progenitor cells (HSPCs) are crucial for lifelong blood cell production. We analysed the cell cycle and cell production rate in HSPCs in murine haematopoiesis. The labelling of DNA-synthesizing cells by two thymidine analogues, optimized for in-vivo use, enabled the determination of the cell cycle flow rate into the G2phase, the duration of the S-phase and the average cell cycle time in Sca-1 + and Sca-1 - HSPCs. The determination of cells with 2n DNA content and labelled during the preceding Sphase was used to establish the cell flow rates in the G1phase. Our measurements revealed a significant difference in how Sca1 + and Sca1 - HSPCs self-renew and differentiate. The division of Sca-1 + progenitors led to the loss of the Sca1 marker in about half of newly produced cells, corresponding to asymmetric cell division. In contrast both Sca-1 - progenitors, arising from mitotic cell division, entered a new round of the cell cycle. This corresponds to symmetric self-renewing cell division. The novel data also enabled us to estimate the cell production rates in the Sca-1 + and in three subtypes of Sca-1 - HSPCs. We focused on adult murine erythroid differentiation in the next part of our study. We introduced an original flow cytometry approach for identifying and studying erythroid progenitor and precursor cells. This approach is based on the changing expression of two cell surface markers, c-Kit (receptor for stem cell factor) and CD71 (transferrin receptor 1) in bone marrow cells deprived of granulocytes, monocytes, lymphocytes and Sca-1 + cells. We identified the early erythroid progenitor cells with BFUE and CFUE potentials within the cell population highly expressing c-Kit. The potential to give rise to BFU-E and CFU-E colonies was lost in the cells highly expressing CD71. Subsequently, erythroid differentiation progressed into the proerythroblasts which expressed c-Kit at a high level. Analysis of the cell cycle revealed that the differentiation of proerythroblasts into basophilic erythroblasts occurs in the course of a single cell cycle, in fact predominantly in the Sphase. During this S-phase, cells maintain the high expression of CD71 but rapidly lose the c-Kit marker and express the erythroid marker Ter119. The dual EdU-BrdU sequential labelling of DNA synthesizing cells, together with the metaphase block induced by colchicine, provide us with unique insights into the dynamics of cell proliferation and differentiation events in early erythroid cells.