Faltusová Kateřina, MSc., PhD

Topic:
The role of stem cells in regeneration of hematopoiesis.
 www.researchgate.net/profile/Katerina_Faltusova
www.researchgate.net/profile/Katerina_Faltusova
Kateřina Faltusová Publications
Altered erythro-myeloid progenitor cells are highly expanded in intensively regenerating hematopoiesis.
K. Faltusová, C-L. Chen , T. Heizer, M. Báječný, K. Szikszai, P. Páral, F. Savvulidi, N. Renešová and E.Necas (2020), Front. Cell Dev. Biol. doi: 10.3389/fcell.2020.00098
Abstract:
Regeneration of severely damaged adult tissues is currently onlypartially understood. Hematopoietic tissue provides a uniqueopportunity to study tissue regeneration due to its well establishedsteady-state structure and function, easy accessibility, wellestablished research methods, and the well-defined embryonic,fetal, and adult stages of development. Embryonic/fetal liverhematopoiesis and adult hematopoiesis recovering from damageshare the need to expand populations of progenitors and stem cellsin parallel with increasing production of mature blood cells. In thepresent study, we analyzed adult hematopoiesis in mice subjected toa submyeloablative dose (6 Gy) of gamma radiation and targeted theperiod of regeneration characterized by massive production ofmature blood cells along with ongoing expansion of immaturehematopoietic cells. We uncovered significantly expandedpopulations of developmentally advanced erythroid and myeloidprogenitors with significantly altered immunophenotype. Theirpopulation expansion does not require erythropoietin stimulationbut requires the SCF/c-Kit receptor signaling. Regeneratinghematopoiesis significantly differs from the expandinghematopoiesis in the fetal liver but we find some similaritiesbetween the regenerating hematopoiesis and the early embryonicdefinitive hematopoiesis. These are in 1) the concomitant populationexpansion of myeloid progenitors and increasing production ofmyeloid blood cells 2) performing these tasks despite the severelyreduced transplantation capacity of the hematopoietic tissues and 3)the expression of CD16/32 in most progenitors. Our data thusprovide a novel insight into tissue regeneration by suggesting thatcells other than stem cells and multipotent progenitors can be offundamental importance for the rapid recovery of tissue function.
Regenerating haematopoiesis resembles embryonic stem cell-independent haematopoiesis. [in czech only]
E.Nečas, K. Faltusová, (2020), Transfuze Hematol. dnes, Online only, p. 1-15. ISSN 1805-4587
Abstract:
Tissue regeneration is a complex and highly orchestrated process leading to the reconstitution of damaged tissue and recovery of its function. Haematopoietic tissue has extensive regenerative potential which is attributed to the presence of haematopoietic stem cells. This paper briefly discusses the current understanding of haematopoietic stem cells and their participation in steady-state haematopoiesis. It also gives an overview of the three phases of embryonic and foetal haematopoiesis preceding the establishment of steady-state adult haematopoiesis. The paper presents the main conclusions drawn from our analysis of intensively regenerating bone marrow following severe damage by ionizing radiation. The research revealed a fundamental role that the developmentally advanced myeloid progenitor cells play in bone marrow regeneration, which occurs during the virtual absence of stem cells and multipotent progenitors. The regeneration induced by progenitors explains why the strong regenerative power of this tissue cannot be transplanted to other subjects with damaged haematopoiesis. A comparison of the rapidly expanding regenerating bone marrow with the expanding foetal liver haematopoiesis showed significant differences between them. However, there is a similarity between intensively regenerating haematopoiesis and embryonic definitive haematopoiesis occurring prior to the emergence of haematopoietic stem cells.
T-lymphopoiesis is Severely Compromised in Ubiquitin-Green Fluorescent Protein Transgenic Mice
K. Faltusová, M. Báječný, T. Heizer, P. Páral, E. Nečas (2020), FOLIA BIOL (PRAHA). vol. 66, no. 2, p 47-59 ISSN: 0015-5500.
Abstract:
Tagging cells of experimental organisms with genetic markers is commonly used in biomedical research. Insertion of artificial gene constructs can be highly beneficial for research as long as this tagging is functionally neutral and does not alter the tissue function. The transgenic UBC-GFP mouse has been recently found to be questionable in this respect, due to a latent stem cell defect compromising its lymphopoiesis and significantly influencing the results of competitive transplantation assays. In this study, we show that the stem cell defect present in UBC-GFP mice negatively affects T-lymphopoiesis significantly more than B-lymphopoiesis. The production of granulocytes is not negatively affected. The defect in T-lymphopoiesis causes a low total mber of white blood cells in the peripheral blood of UBC-GFP mice which, together with the lower lymphoid/myeloid ratio in nucleated blood cells, is the only abnormal phenotype in untreated UBC-GFP mice to have been found to date. The defective lymphopoiesis in UBC-GFP mice can be repaired by transplantation of congenic wild-type bone marrow cells, which then compensate for the insufficient production of T cells. Interestingly, the wild-type branch of haematopoiesis in chimaeric UBC-GFP/wild-type mice was more active in lymphopoiesis, and particularly towards production of T cells, compared to the lymphopoiesis in normal wild-type donors.
Current understanding of the haematopoietic tissue. [PDF in czech only]
E.Necas, K. Faltusová, (2019), Československá fyziologie vol. 68 no.2, str. 57 - 67, ISSN: 1210 - 6313
Abstract:
Haematopoietic tissue carries out the life long production of blood cells. The quantitative and qualitative aspects of blood cell production are summarised in this short review. The haematopoietic tissue is the best researched tissues regarding the role of stem and progenitor cells in tissue homeostasis and function. Advanced experimental methods, especially cell transplantation and flow cytometry, enable research into the functional organisation of haematopoietic tissue beyond the limits present in other tissues. The review focuses upon the haematopoietic stem cell, the developmental hierarchy in blood cell formation and the role of stroma in the support and control of blood cell production. A brief overview of embryonic and foetal haematopoiesis is also provided.
Stem Cell Defect in Ubiquitin-Green Fluorescent Protein Mice Facilitates Engraftment of Lymphoid-Primed Hematopoietic Stem Cells.
Faltusová, K., Szikszai, K., Molík, M., Linhartová, J., Páral, P., Šefc, L., Savvulidi, F. and Nečas, E. (2018), STEM CELLS. doi: 0.1002/stem.2828.
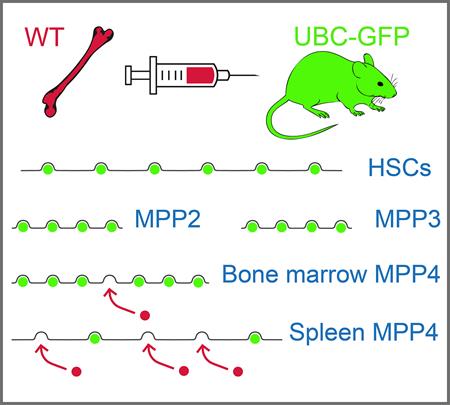 Abstract: Abstract:Transgenic mice expressing green fluorescent protein (GFP) are useful in transplantation experiments. When we used ubiquitin-GFP (UBC-GFP) transgenic mice to study the availability of niches for transplanted hematopoietic stem and progenitor cells, the results were strikingly different from the corresponding experiments that used congenic mice polymorphic in the CD45 antigen. Analysis of these unexpected results revealed that the hematopoiesis of UBC-GFP mice was outcompeted by the hematopoiesis of wild-type (WT) mice. Importantly, UBC-GFP mice engrafted the transplanted bone marrow of WT mice without conditioning. There was a significant bias toward lymphopoiesis in the WT branch of chimeric UBC-GFP/WT hematopoiesis. A fraction of immature Sca-1+ cells in the spleen of UBC-GFP mice expressed GFP at a very high level. The chimeric hematopoiesis was stable in the long term and also after transplantation to secondary recipient mice. The article thus identifies a specific defect in the hematopoiesis of UBC-GFP transgenic mice that compromises the lymphoid-primed hematopoietic stem cells in the bone marrow and spleen. |
Hematopoiesis Remains Permissive to Bone Marrow Transplantation After Expansion of Progenitors and Resumption of Blood Cell Production.
Báječný, M., Chen C.-L.,Faltusová, K., Heizer T., Sziskszai K., Páral P., Šefc, L., and Nečas, E. (2021)., Front. Cell Dev. Biol., https://doi.org/10.3389/fcell.2021.660617
Cell cycle and differentiation of Sca-1 + and Sca-1 − hematopoietic stem and progenitor cells.
Páral, P., Faltusová, K., Molík, M., Renešová, N., Šefc, L., and Nečas, E. (2018)., Cell Cycle 17, 1979–1991. doi:10.1080/15384101.2018.1502573
Low c-Kit Expression Level Induced by Stem Cell Factor Does Not Compromise Transplantation of Hematopoietic Stem Cells.
Chen C-L, Faltusova K, Molik M, Savvulidi F, Chang K-T, Necas E. Biol Blood Marrow Transplant 2016, 22:1167–1172.
Hematopoietic stem cells survive circulation arrest and reconstitute hematopoiesis in myeloablated mice.
Michalova J, Savvulidi F, Sefc L, Faltusova K, Forgacova K, Necas E: Biol Blood Marrow Transplant 2011, 17:1273–81.
Kateřina Faltusová Posters
Lack of evidence for active role of hematopoietic stem cells in intensive bone marrow regeneration.
Faltusová K., Heizer T., Báječný M., Nečas E.; Aegean Conferences, International Conference on Stem Cells, Crete 2019
Stem cell defect in UBC-GFP mice facilitates engraftment of lyphoid- primed hematopoietic stem cells.
Faltusová K., Szikszai K., Molík M., Linhartová J., Páral P., Šefc L., Savvulidi F., Nečas E.; EMBL, Hematopoietic Stem Cells: From the Embryo to the Aging Organism, Heidelberg 2018
A latent defect in lymphoid-biased hematopoietic stem cells in UBC-GFP transgenic mice.
Katerina Faltusova, Katarina Szikszai, Martin Molik, Filipp Savvulidi, Emanuel Necas. ISEH, Frankfurt, 2017.
Late myeloid progenitors regenerate haematopoiesis in submyeloablatively irradiated mice.
Katerina Faltusova, Petr Paral, Martin Molik, Chia-Ling Chen,Ludek Sefc, Emanuel Necas. Lyon, 2016.
c‑Kitlow progenitors regenerate damaged hematopoiesis.
Katerina Faltusova, Petr Paral, Martin Molik, Chia-Ling Chen, Ludek Sefc, Emanuel Necas. EMBL, Heidelberg, 2016.
Reconstitution of hematopoiesis from a small number of repopulating cells surviving in irradiated mice.
Katerina Faltusova, Katarina Szikszai-Forgacova, Martin Molik, Filipp Savvulidi, Petr Paral, Ludek Sefc, Emanuel Necas. ISSCR, Stockholm, 2015.
Kateřina Faltusová PhD thesis
The role of stem and progenitor cells in regeneration of hematopoietic tissue
Prague, 2021
Abstract:
Tissue regeneration is a complex and highly orchestrated process dependent on cells with the potential to restore structures and functions and on controlling factors from the tissue microenvironment. Hematopoietic tissue has a high ability to regenerate, which is attributed to the presence of stem cells, but the regeneration of severely damaged adult tissue is still only partially understood. Hematopoietic tissue provides a unique opportunity to study tissue regeneration due to its well-established steady-state structure and function, easy accessibility, advanced research methods, and well-defined embryonic, fetal, and adult stages of development. Embryonic/fetal liver hematopoiesis and adult hematopoiesis recovering from damage share the need to expand populations of progenitors and stem cells in parallel with increasing production of mature blood cells.
We analyzed adult hematopoiesis in mice subjected to a submyeloablative dose (6 Gy) of gamma radiation, in which only a few cells with reconstituting capacity survived. We targeted the period of regeneration characterized by the renewed massive production of mature blood cells and the ongoing expansion of immature hematopoietic cells. Cells from the top of the hematopoietic hierarchy, hematopoietic stem cells, and multipotent progenitors are almost missing in this period of hematopoiesis regeneration. We uncovered significantly expanded populations of developmentally advanced erythroid and myeloid progenitors with significantly altered immunophenotype and with the ability for intensive proliferation. These immature hematopoietic cells differ from the progenitor cells present in normal bone marrow by the decreased expression level of the c-Kit receptor for stem cell factor, the expression of Sca-1 antigen also in the cells which express transferrin receptor 1 (CD71), by expression of CD16/32 in most of the cells, and by altered expression of CD41. These progenitors activated the erythroid developmental program independently from erythropoietin production. Despite decreased expression of the c-Kit receptor, progenitors require effective stimulation by stem cell factor (SCF) for their expansion.
Hematopoietic stem cells, defined by their ability to reconstitute destroyed hematopoiesis in the host, were reduced to 1 – 2 % of their normal number in the intensively regenerating hematopoiesis.
It was shown that the early reconstitution of hematopoiesis from transplanted cells that were not exposed to radiation gives rise to populations of altered progenitors, which are similar to those identified in the bone marrow regenerating from endogenous cells surviving exposure to ionizing radiation.
Regenerating hematopoiesis differs significantly from the expanding hematopoiesis in the fetal liver by the virtual lack of stem cells and different immunophenotypes of progenitor cells
The data presented in this study provide a novel insight into tissue regeneration by suggesting that cells other than stem cells and multipotent progenitors can be of fundamental importance for the rapid recovery of tissue function, and the regenerating adult hematopoiesis shares some features with the embryonic hematopoiesis preceding the development of stem cells.